Using whole animal physiology, in vivo electrical stimulation of the heart and echocardiography, we study the predisposition of the heart to develop lethal arrhythmias and its association with diastolic dysfunction (heart failure with preserved ejection function, HFpEF). On the cellular level, we use isolated cardiomyocytes from these animal models, for cellular electrophysiology studies and direct measurements of calcium transients to measure abnormalities of repolarization, dysfunction and distribution of ion channels, and calcium dyshomeostasis in the predisposition to the development of ventricular arrhythmias. Using molecular biological tools, we study the role of second messenger signaling in the regulation of ion channel activity in the predisposition to ventricular arrhythmias, and development of diastolic dysfunction. We also have an interest in the role of Toll-Like receptors in the pathogenesis and treatment of mouse models for abdominal aortic aneurysms.
Current projects include:
The MADIT trial used programmed ventricular stimulation (vstim) to determine which patients who have had an MI are at risk for spontaneous ventricular tachycardia (VT) and potential sudden cardiac death and, therefore candidates for Implantable Cardiac Defibrillators (ICD) (Moss et al. 2002). Mark Aronovitz and members of the MCRI created a catheter specifically sized to perform vstim on mice. We are one of the few labs to use this technique and develop protocols to determine whether mice were inducible for VT and whether inducibility would serve as a surrogate to predict the development of spontaneous VT following myocardial infarction. We are using this approach to establish new mechanisms of arrhythmogenesis and identify molecular targets for the detection and prevention of VT.
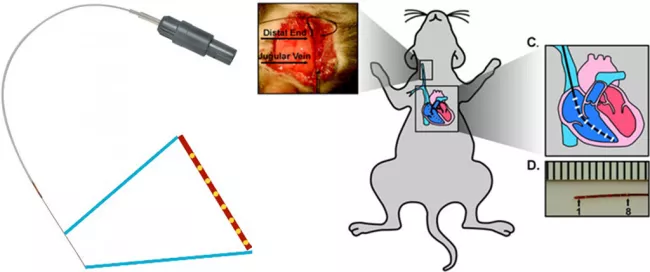

We also investigate the cellular mechanisms underlying pro-arrhythmic substrate, employing different mouse models, including diabetic heart disease, metabolic heart syndrome, hypertrophic cardiomyopathy and heart failure. Our research approach combines multiple disciplines, including cellular electrophysiology, single-cell RT qPCR, immunohistochemistry, biochemistry and genetic approaches. Our research focus includes: 1) characterizing cardiac ion channels, and lysosomal ion channels, exploring their roles as contributors to pro-arrhythmic substrates. We are interested in exploring the functional characteristics and molecular profiles of these channels in both physiological and cardiovascular diseases. We also investigate their significance in shaping the action potential and their potential contributions as pro-arrhythmic substrates; 2) Investigating second messengers including cGMP and phosphodiesterase in these molecular changes. We seek to highlight its therapeutic potential in the development and treatment of arrhythmias.
Building upon our findings from mouse models, we seek to develop non-invasive metrics for detecting the risk for sudden cardiac death, establish a standardized protocol and validate its accuracy for the prevention and early detection of those life-threatening events.
Given that the incidence of sudden arrhythmic death is markedly increased in diabetics we developed a mouse model for post-myocardial infarction (post-MI) ventricular tachycardia (VT) in the diabetic heart and determined the antiarrhythmic effect of statins in this model.
Using the Akita mouse, a model for type 1 diabetes we demonstrated that following a coronary artery ligation mice developed ventricular tachycardia over a 30-hour period and that this arrhythmia was markedly suppressed in mice treated with the HMG CoA reductase inhibitor pravastatin. The development of VT was shown to be associated with abnormalities of Ca uptake and release in cardiomyocytes isolated from these mice, which were reversed by pravastatin treatment.

We have developed a unique approach for the use of programmed atrial stimulation for the study of the mechanisms of atrial fibrillation in mouse models for metabolic heart disease. Using isolated atrial cardiomyocytes, we are studying the development of abnormalities of repolarizing currents in the pathogenesis of atrial fibrillation in these mice.
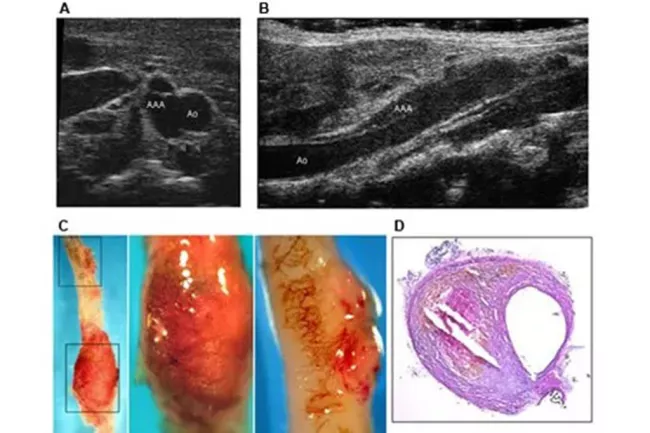
We have demonstrated that statins regulate angiogenesis both in vivo and in vitro via the control of the activity of the small GTP-binding protein Rho. Our current studies deal with the relationship between hypertension, hyperlipidemia and angiogenesis and the role of statins in regulating the development of abdominal aortic aneurysms in a mouse model. More recently we have initiated studies of the role of innate immunity and Toll-Like receptors on the distribution of T-cells to the abdominal aorta in the development and progression of AAAs.
Lab members
- Xuehong Cao, MD, PhD
- Yali Zhang, MD, PhD
- Irakli Tadua, MD